Deep Dive: Why measuring alkalinity is not the same as quantifying HCO₃⁻
How to avoid confusion about the seemingly straightforward use of alkalinity to quantify CO₂ removal
Enhanced rock weathering (ERW) is a carbon dioxide removal (CDR) method where crushed rocks are added to the soil. In the presence of water, these rocks will dissolve through a chemical reaction that changes CO₂ into HCO₃⁻ and CO₃²⁻. The most straightforward way to measure how much CO₂ is removed would thus be measuring the increase of HCO₃⁻ in the soil water. However, HCO₃⁻ is a parameter that can not be directly measured but only indirectly calculated through other measurements. Alkalinity is a soil water parameter related to the HCO₃⁻ concentrations that is often used to get insights into the CDR efficiency of ERW.
Since the publication of our blogpost and paper ‘’ CDR Measurement for ERW via Alkalinity in Leachate (Data From Our Greenhouse Experiment, Part 2)’’ we have noticed that there might be some confusion about the correlation between alkalinity measured through acid titration and the bicarbonate (HCO₃⁻) concentration of a soil water/leachate sample. In short: Alkalinity does not equal HCO₃⁻, but under specific conditions one can assume that it closely represents it. It's an approximation of the HCO₃⁻ concentration under certain assumptions, not an exact chemical concentration.
Here is what we initially wrote about the titration procedure to measure alkalinity in our leachate soil water samples:
“With the titrator, sulfuric acid (1.6 N) is added in tiny drops to the sample until the indicators change colours at around pH 4.3. According to the HACH titrator instructions, this amount of added acid can be expressed as CaCO₃ mg/l. This result is converted into mg/l HCO₃⁻ by multiplying with a factor of 1.22 for conversion from CaCO₃ to HCO₃⁻ . In order to convert this number to micromol we divide by 61, the molar weight of HCO₃⁻ . Note that other species may contribute to alkalinity (i.e. the acid buffering capacity) but in most solutions we deal with it is a safe assumption that HCO₃⁻ is the major acid buffer. … To make this text better readable we will from now on use the word “alkalinity” for “titration alkalinity” as explained above.”
The final data resulting from the acid titration measurement is hence the alkalinity in meq/L (mmol(eq)/L) and this represents the equivalent of the charge that buffered the added acid until we reach the pH of 4.3. In seawater, the alkalinity is mostly made up of carbonate ions HCO₃⁻ and CO₃²⁻ besides B(OH)4−, H+, OH−, etc.). In freshwater such as rivers or soil water, it may also include phosphates, metal-organic complexes,... But for a qualitative assessment and comparison between different soils with treatments and their controls (pots with only soil), the alkalinity of the leachate samples can be assumed to represent mostly anions of the carbonate system with only minor other anions. This is assumption #1.
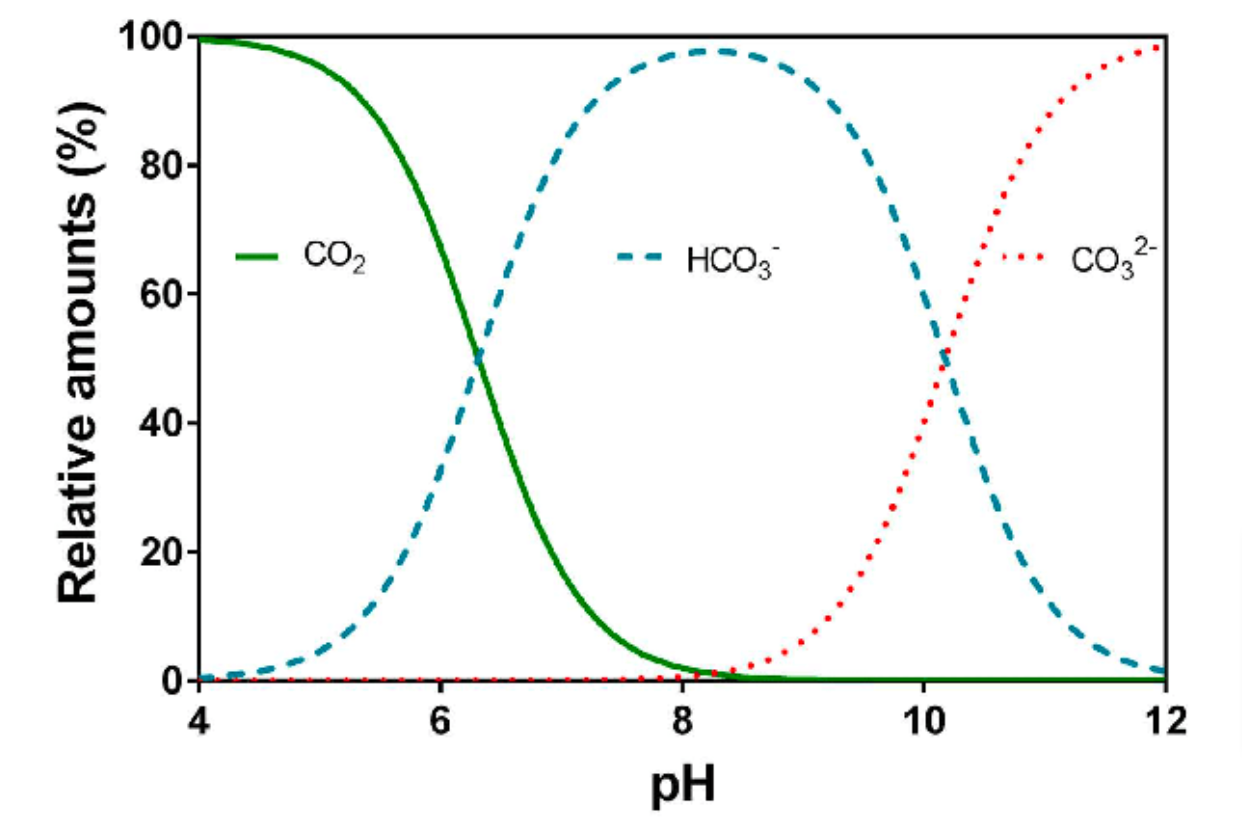
Bjerrum Plot, Source: Pedersen et al. (2013), from our Dec 2023 paper
Besides the fact that alkalinity is largely, but not exclusively, representing the carbonate system, one also needs to take into account the pH as this dictates which carbon anions are present. The dissolved inorganic carbon (DIC) system involves the following reactions: CO₂+H₂O combines to H₂CO₃ which can dissociate into HCO₃⁻ + H⁺ and in a next step into CO₃²⁻ + 2 H⁺ depending on the water pH. In the Bjerrum plot (on the right), taken from our paper mentioned above, one can see how at different pH values, the same amount of DIC will represent different HCO₃⁻- concentrations. Only at a soil water pH 6.5-8 is the DIC mainly present as bicarbonate anions and one could assume the carbonate system to be made up of only HCO₃⁻. This is assumption #2.
Number of occurrences of pH values in 2023, colors symbolize soils, from our Dec 2023 paper
The second graph with the distribution of all pH measurements done in 2023 in our greenhouse experiment shows that most of them are indeed in this pH range. So under assumption #1 we could thus interpret the measured alkalinity as roughly the HCO₃⁻ contents. But in reality, the HCO₃⁻ contents of the leachate sample will be lower than the alkalinity as this may also include CO₃²⁻ and other anions. There are other ways than titration to quantitatively obtain the HCO₃⁻ concentration of a solution and we will write a more detailed text on this in the future.
Generally we are not interested in the absolute composition of the leachate water of a particular soil-rock treatment, but in the relative difference that we might observe between the treatment and its respective control (same soil without rock dust). We hypothesise that if enhanced weathering is occurring in the treatment, we will see an increase in the HCO₃⁻ concentration of its leachate. Hence we subtract the titration measurement of the control leachate water from that of the corresponding treatments, and refer to the results as ‘additional alkalinity’ as mentioned in the blog article (CDR Measurement for ERW via Alkalinity in Leachate (Data From Our Greenhouse Experiment, Part 2)).
“We assume that one mol of this additional alkalinity (compared to control) corresponds to one mol of bicarbonate (HCO₃⁻) which was formed during the weathering process and which was formerly one mol of CO₂ taken from the atmosphere some time earlier. This reasoning includes the assumption that all alkalinity resulting from natural soil background (organic alkalinity, pH, carbonate minerals) is cancelled out by the subtraction of the control.”
This is assumption #3.
Any inaccuracies resulting from assumption #1 (there were other ions contributing to alkalinity apart from the carbonate system) or assumption #2 (pH of the solution is such that not all DIC is present as HCO₃⁻) are thus assumed to be the same in control and in treatment under assumption #3. Under these three assumptions, the relative difference in leachate alkalinity between a treatment and its control can be interpreted as the additional HCO₃⁻ in the treatment leachate. In reality, however, the soil system is immensely complex and there will likely be differences in pH and alkalinity between control and treatment which are not linked to changes in HCO₃⁻. Hence, the alkalinity of the control (or of the treatment) is even less likely to straightforwardly represent the HCO₃⁻ concentration of this solution.
In short: When one carries out titrations in EW experiments or trials, one should not simply connect the resulting alkalinity to the solution’s HCO₃⁻ concentration. Instead, it is better to express your results in meq/l of titrated alkalinity which includes the HCO₃⁻ concentration. Only when in your sample (1) the alkalinity is almost exclusively made up of the carbonate system and (2) the pH is in the range where there is mostly HCO₃⁻ , you could (3) subtract the alkalinity of a control from the alkalinity of a treatment and interpret the result as HCO₃⁻ difference due to rock dust addition.